This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful. More information in our Privacy Policy
PATROLS
Physiologically anchored tools for realistic nanomaterial hazard assessment
Principal investigator: Anna Luisa Costa
Involved personnel: Magda Blosi, Carlo Baldisserri, Andrea Brigliadori, Antonio Crimaldi, Lara Faccani, Davide Gardini, Simona Ortelli, Marina Serantoni, Felice Carlo Simeone, Ilaria Zanoni
Starting date: 01/01/2018
Duration: 48 months
Total funding: 13 034 497,50 €; ISTEC: 441 845,00 €
Grant Agreement No.: 760813
Call: H2020-NMBP-2017-two-stage
Topic: NMBP-29-2017 – Advanced and realistic models and assays for nanomaterial hazard assessment
Coordinator: Shareen Doak (Swansea University, UK)
Consortium: 24 partners
Project Officer: Achielleas Stalios
Official website: https://www.patrols-h2020.eu/
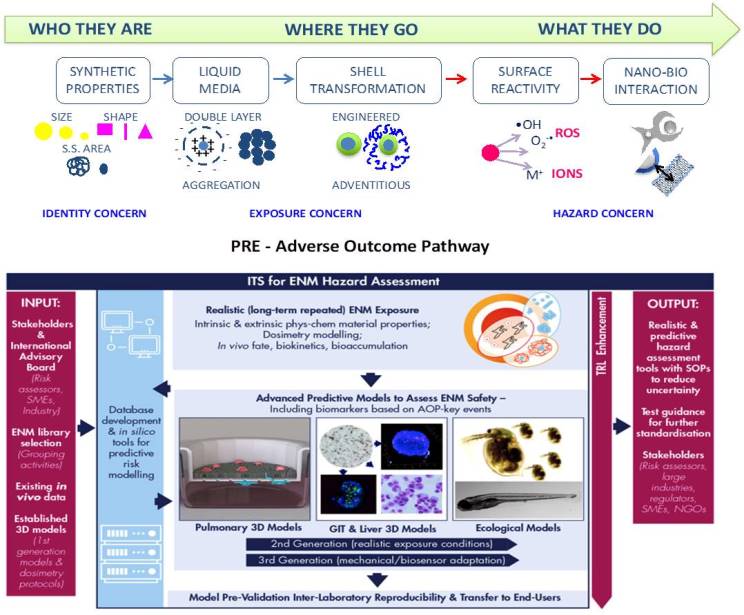
Classical hazard testing strategies to define the human and environmental health impact of engineered nanomaterials (ENM) commonly apply unrealistic acute, high-doses to models that do not reflect the in vivo environment. Furthermore, existing in vitro and in silico hazard detection methods are not accurately predictive. PATROLS address these limitations by establishing and standardizing the next generation of advanced safety assessment tools for improved prediction of the adverse effects caused by chronic ENM exposure in human and environmental systems.
PATROLS will deliver:
1) physiologically representative multi-cellular in vitro 3D lung, gastrointestinal tract and liver models
2) cross-species models integrating human and environmental safety testing
3) innovative ecotoxicity bioassays in several organisms across a food chain.
4) robust in silico models for dosimetry, interspecies toxicity extrapolation and hazard prediction.
ENM characterization under physiologically relevant experimental conditions will be integral to this realistic, exposure driven strategy.
ISTEC will lead WP1-ENM acquisition, identification and exposure assessment addressing physicochemical characterization and experimental design strategies in support to bioassays:
1) providing primary (intrinsic) and system dependent (extrinsic) ENM characteristics in complex biological matrices, under realistic long-term exposure conditions, using existing data and a range of complementary analytical techniques.
2) providing ENM identity with additional information on their fundamental behavior and possible changes, necessary for read-across justification.
Partners:
Swansea University (SU)
The University of Exeter (UNEXE)
Universite de Fribourg (AMI), Heriot-Watt University (HWU)
Universiteit Leiden (UL)
Universita Di Pisa (UNIPI)
Université Catholique Louvain (LTAP)
Universite de Namur ASBL (UNamur)
Danmarks Tekniske Universitet (DTU)
QSAR Lab Spolka Z Organiczona (QSAR)
Harvard Global Research and Support Services, Inc (Harvard)
University of South Carolina (USC)
University Det Nationale Forskningscenter Forarbejdsmiljo (NRCWE)
Rijksinstituut voor Volksgezondheid en Milieu (RIVM)
Korea Research Institute of Standards and Science (KRISS)
Institute of Occupational Medicine (IOM)
Consiglio Nazionale Delle Ricerche (ISTEC)
Institut Fur Umweltmedizinische Forschung An Der Heinrich-Heine-Universitat Dusseldorf (IUF)
BASF SE (BASF)
INSPHERO AG (InSphero)
MISVIK BIOLOGY OY (Misvik)
Nanotechnology Industries Association (NIA)
European Research Services (ERS), Medical Research Council (NC3Rs)
