This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful. More information in our Privacy Policy
Smart biomaterials for controlled drug-release
Principal Investigators: Anna Tampieri, Monica Sandri, Simone Sprio, Michele Iafisco
Personnel involved: Elisabetta Campodoni, Massimiliano Dapporto, Francesca Carella, Marta Tavoni, Lorenzo Degli Esposti
Bone diseases can have both traumatic and metabolic causes. The latter refer to alterations in the physiological bone metabolism, triggered by infections and inflammations (e.g. osteomyelitis) or neoplastic processes (e.g. osteosarcomas).
Orthopedic surgery frequently requires invasive access for the treatment of many pathologies, raising the risk of infections by pathogens (e.g. bacteria) especially in the hospital environment. In particular, pathogens can colonize both the operating field and the surface of the implanted biomaterials, compromising the surgical outcome.
For this reason, antibiotics are often administered as pre-, peri- and post-operative treatments with the aim to reduce the risk of infection.
In recent years, Scientific Research has been trying to face a growing demand for implantable biomaterials able to convey drugs in situ, that is conveyed by the implant itself, thus eliminating the undesirable effects deriving from high systemic administrations.
The biomaterials developed by ISTEC-CNR for bone regeneration, are characterized by high biocompatibility and biomimetism, that is, they are able to mimick the chemico-physical, morphological and mechanical features of the bone tissue, while also excellent candidates as smart controlled drug-release systems.
Several approaches can be pursued to carry out the loading of the drug in a device, including the introduction of drug-loaded components, and the surface adsorption of the drug by immersion in pharmaceutical solutions.
The biomaterials selected by ISTEC-CNR to implement such drug-loading and drug-release studies are:
- bone cements, in the form of self-hardening mixtures for injection in minimally invasive surgery;
- macroporous scaffolds selected by forming techniques such as replica or direct foaming;
- hybrid scaffolds made of self-assembled type I collagen fibers mineralized with magnesium-doped hydroxyapatite nanoparticles
The drugs selected for controlled release studies include antibiotics, in particular Vancomycin (an antibiotic commonly used to treat infections by Gram + bacteria, such as Staphylococcus Aureus, responsible for osteomyelitis) and Gentamicin, a broader-spectrum inhibitor of protein synthesis, and also specific drugs to treat osteomyelitis.
Other drugs currently studied include anticancer drugs for the treatment of neoplastic diseases such as osteosarcoma. Following the surgical removal of the tumor mass, the two main issues are the replacement of critical-size bone defects and also the successfully eradication of neoplastic cells.
In the absence of specific pharmacological features, an implanted biomaterial could not exclude the formation of cancer recurrences. In this context, the local anticancer drugs release from biomaterials can be strategic towards the complete post-surgical bone regeneration. The anticancer drugs selected for these studies include doxorobucin, methotrexate or more specific molecules for target therapy such as anti-RANKL and anti-mTOR antibodies.
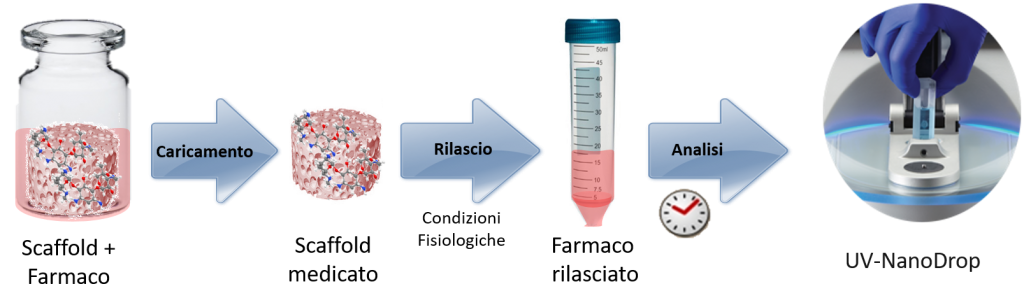
The preliminary results obtained from these research as promising and open new perspectives for the development of medicated biomaterials.
Schematic representation of the drug-loading on the biomaterial surface, followed by drug-release kinetics.
Equipment and processes
The drug-loading can be pursued by multiple strategies, including surface adsorption by immersion in a medicated solution or the introduction into the biomaterial of nanoparticles or polymer or hybrid microcapsules previously functionalized with the drug.
The chemical structure of the drugs is very relevant towards the choice of suitable analytical techniques for the quantification of their release. One of these techniques is represented by visible UV spectroscopy (NanoDrop™ OneC Microvolume UV-Vis Spectrophotometer).
In ISTEC-CNR, drug release studies are conducted in liquid media able to mimick the physiological composition of human body fluids.
Main Collaborations
- Finceramica
- Greenbone
- IRST
- IOR
- IRET
Projects
- BIOBOS
- DINAMICA
