This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful. More information in our Privacy Policy
Ultra refractory multi-element ceramics
Stabilization and development of ultra-refractory ceramics via entropy engineering
Principal investigator: Frédéric Monteverde
Involved personnel: Federico Saraga
The “compositional disorder” can be engineered into multi-element compositions by populating specific sub-lattices of high symmetry crystalline structures, such as BCC or FCC, with multiple chemically distinct cations. The resulting compounds often lead to undiscovered crystalline compounds where anions and cations interact with each other giving rise to new chemical bonds.
Thermodynamic models of these multicomponent systems show that the entropic contribution to Gibbs free energy predominates over the thermodynamic one and can control reversible solid state transformations between a multiphase state and a monophasic state. The random and homogeneous distribution of atoms contributes to the stabilization of the new structure.
The entropy engineering offers a powerful strategy to
- conceive and discover new crystalline phases;
- investigate unexplored opportunities for the engineering of functional properties.
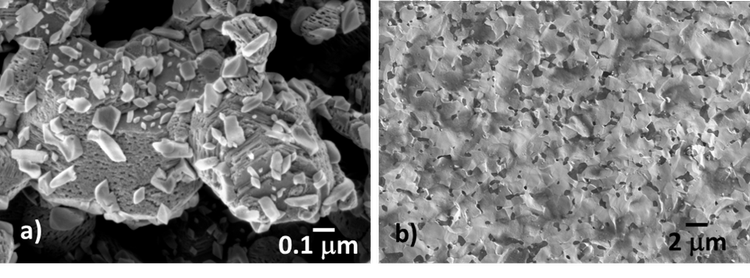
Multi-element ultra-refractory ceramics, better historically recognized as high entropy materials, belong to the class of diborides and carbides of transition metals, and catalyze an intense fundamental research that spans from the synthesis of single phases and converges to the manufacturing of sintered pieces. The entropic term can be managed to form various solid solutions of new compounds.
The micro-hardness of these multi-component solid solutions already showed to make a qualitative leap with respect to that of the individual components.
Equipment and processes
Publications & Patents
- F. Monteverde, F. Saraga, Entropy stabilized single-phase (Hf,Nb,Ta,Ti,Zr)B2 solid solution powders obtained via carbo/boro-thermal reduction, Journal of Alloys and Compounds Volume 824, 25 May 2020, 153930
